卡介苗膀胱灌注预防中、高危非肌层浸润性膀胱癌复发的疗效及并发症分析
2019年1月
中华儿科杂志,第40卷第1期 第14页-第19页
孙卫兵,刘志宇,李泉林,宋希双,孔祥波,王春喜,张奇夫,祝清国,李长福,徐万海,于广海,张诚,杨进益,宋天家,赵积烨,付启忠,王立新,丁全忠,蔡学辉,孔垂泽
膀胱癌的发病率居我国男性泌尿生殖系统恶性肿瘤首位。初诊时约75%的原发膀胱癌属于非肌层浸润性膀胱癌(non-muscle invasive bladder cancer,NMIBC)。经尿道膀胱肿瘤切除术(transurethral resection of bladder tumor,TURBT)是NMIBC的主要治疗手段[1]。NMIBC术后复发率为50%~85%,其中30%~55%出现肿瘤进展[2]。术后膀胱内灌注治疗,可降低肿瘤复发率,提高患者生存率。膀胱灌注药物主要包括化疗药物(如丝裂霉素、表阿霉素等)和免疫制剂[如卡介苗(Bacillus Calmette Guerin vaccine,BCG)和干扰素等]两大类。1976年美国文献首次报道BCG用于预防NMIBC术后复发具有显著的疗效[3],在预防表浅性膀胱癌特别是T1G3肿瘤和原位癌TURBT术后复发方面疗效显著[4]。欧洲泌尿外科学会(EAU)指南推荐针对不同复发风险的患者给予相应的治疗方案:低危组TURBT术后选择以丝裂霉素C为代表的膀胱内灌注化疗,中、高危组选用BCG膀胱内灌注免疫治疗[1]。
国产BCG已于2013年12月正式上市,目前国内对其疗效及并发症的系统评价较少。我们回顾性分析2013年12月至2016年10月东北三省18家医院共276例应用BCG膀胱灌注治疗的中、高危NMIBC患者的临床、病理资料,观察BCG膀胱灌注治疗后对疾病复发进展的影响,以及并发症发生情况。
本研究分为两个部分
第一部分:回顾性分析11家医院共106例中、高危NMIBC患者的病例资料。大连医科大学附属第二医院32例,中国医科大学附属第一医院20例,大连医科大学附属第一医院17例,吉林大学附属第一医院11例,吉林大学附属中日联谊医院11例,吉林省肿瘤医院4例,哈尔滨医科大学附属第二医院3例,哈尔滨医科大学附属第三医院3例,哈尔滨医科大学附属第四医院2例,大连市中心医院2例,哈尔滨医科大学附属第一医院1例。男83例(78.3%),女23例(21.7%)。平均年龄(66.7±11.7)岁。初发肿瘤73例(68.9%),复发肿瘤33例(31.1%)。肿瘤分期:T1期86例(81.1%)、Ta期20例(18.9%)。肿瘤类型:低级别浸润性尿路上皮癌34例(32.1%),高级别浸润性尿路上皮癌72例(67.9%);其中合并原位癌6例(5.7%),不合并原位癌100例(94.3%)。高危NMIBC 97例(91.5%),中危9例(8.5%)。肿瘤单发45例(42.5%),多发61例(57.5%);肿瘤直径≥3 cm 10例(9.4%),<3 cm 96例(90.6%)。除外同时合并上尿路肿瘤或既往上尿路肿瘤者;首次TURBT切除不完全者(除外Ta G1、低级别的肿瘤);肌层浸润性膀胱尿路上皮癌(≥T2期)患者,或既往有肌层浸润性膀胱尿路上皮癌病史者;膀胱镜随访不规律者;既往泌尿生殖系结核病史或不能有效控制的尿路感染者;TURBT术后未行膀胱即刻灌注治疗的患者。
第二部分:在第一部分研究基础上,增加了被第一部分研究剔除,但符合BCG治疗标准且接受BCG膀胱灌注治疗的患者资料,观察治疗相关并发症发生情况及患者依从性等。共276例患者,大连医科大学附属第二医院123例,大连医科大学附属第一医院68例,中国医科大学附属第一医院20例,吉林大学附属第一医院11例,吉林大学附属中日联谊医院11例,大连市金州区第一人民医院8例,大连市友谊医院8例,大连市中心医院5例,吉林省肿瘤医院4例,大连市旅顺口区人民医院4例,哈尔滨医科大学附属第二医院3例,哈尔滨医科大学附属第三医院3例,哈尔滨医科大学附属第四医院2例,大连大学附属新华医院2例,哈尔滨医科大学附属第一医院1例,大连大学附属中山医院1例,解放军第210医院1例,大连瓦房店市中心医院1例。男211例(76.5%),女65例(23.5%)。平均年龄(68.3±10.3)岁。其中,中、高危NMIBC 263例(95.3%),肾盂/输尿管癌术后膀胱内再发中、高危NMIBC 8例(2.9%),肾盂/输尿管癌同时合并中、高危NMIBC 5例(1.8%)。
所有276例患者术后2周接受BCG(成都生物制品研究所有限公司)膀胱灌注治疗:灌注剂量为120 mg/次,诱导期持续6周,每周1次BCG膀胱灌注治疗,有或没有维持灌注。维持灌注包括3次强化治疗(每周1次BCG膀胱灌注治疗),然后每月1次维持灌注治疗。随访时间≥6个月,观察肿瘤复发及进展情况。术后前2年每3个月1次膀胱镜检查,第3年开始每6个月随访1次,随访5年后每年随访1次。每年对上尿路情况进行1次影像学评估。终点情况:患者膀胱肿瘤复发再次行膀胱肿瘤手术(经尿道手术、膀胱部分切除术或膀胱全切除术);患者不能耐受BCG治疗或出现严重并发症而终止BCG灌注治疗。根据WHO推荐的药物毒副作用及分级标准,将BCG膀胱灌注后并发症分成4级[5,6],记录本研究276例患者的并发症情况。
肿瘤复发定义:膀胱镜活检或诊断性TUR病理结果证实为肿瘤,或者随访过程中尿脱落细胞学检查阳性。肿瘤进展定义:复发肿瘤的分期/分级升高,或伴发原位癌以及肿瘤出现上尿路、前列腺部尿道及远处转移。
采用SPSS 13.0统计软件处理数据。计量资料采用Mean±SD表示,组间比较采用两独立样本t检验;计数资料采用例数表示,组间比较采用χ2检验。采用寿命表法推算中位生存时间。采用单因素分和多因素分析影响BCG膀胱灌注治疗后肿瘤复发的危险因素。以P<0.05为差异有统计学意义。
本研究第一部分106例中,BCG膀胱灌注次数平均(13.4±4.0)次。术后随访6~29个月,平均随访时间(13.6±5.7)个月,中位随访时间12个月。肿瘤复发9例(8.5%),其中2例(1.9%)出现进展;无复发97例(91.5%),无复发时间(13.5±5.7)个月。BCG灌注治疗后,73例初发肿瘤者中4例(5.4%)复发,33例复发肿瘤者中5例(15.1%)再次复发。术后复发时间间隔为3~13个月,中位值8个月。患者1年无复发生存率为91.5%(95%CI 86.2~96.8),患者累积无复发生存曲线见
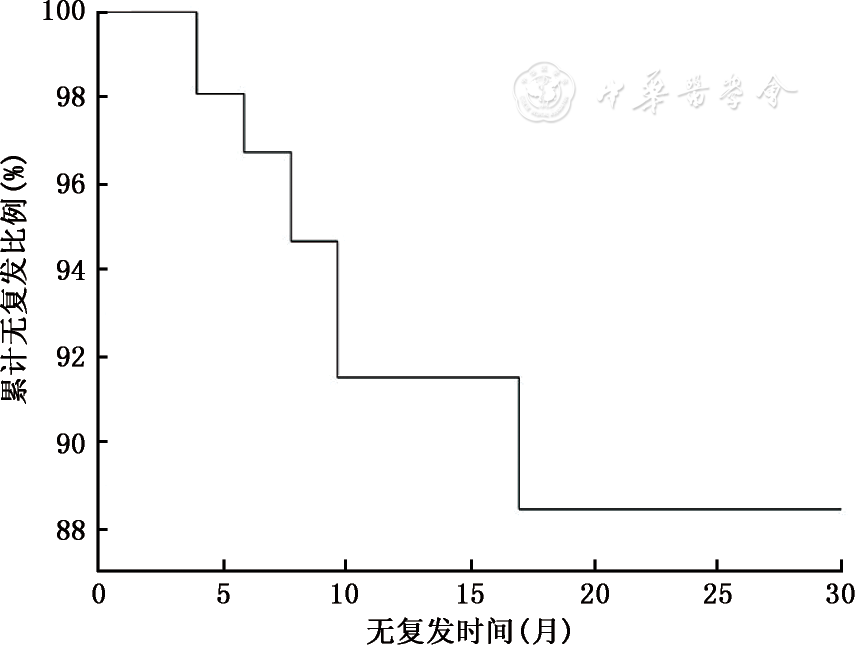
单因素分析结果见
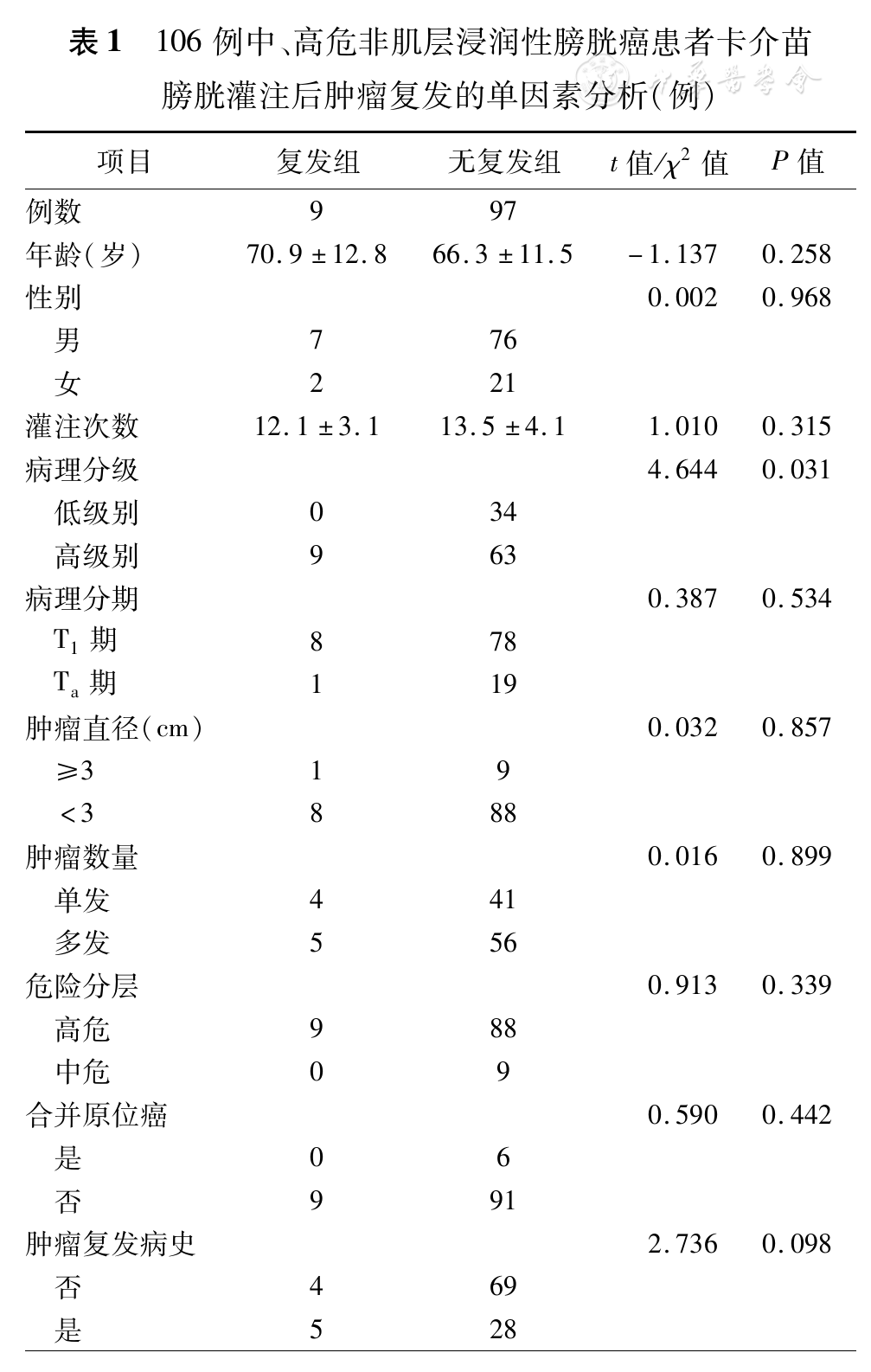
第二部分276例中,177例(64.1%)发生BCG灌注相关并发症,Ⅰ/Ⅱ级并发症166例(60.1%);Ⅲ/Ⅳ级并发症11例(4.0%),分别为全身毒性反应1例、重度尿频及血尿持续≥1周4例、药物性膀胱炎合并肾盂积水1例、体温>39℃持续≥3 d 3例、BCG诱发膝关节炎和附睾炎各1例。36例(13.0%)中途停药,停药原因分别为经济原因9例(3.3%)、出现Ⅲ/Ⅳ级并发症11例(4.0%)、原发病复发或进展更换治疗方案12例(4.4%)、原因不明4例(1.5%)。停药时间为BCG灌注次数<6次30例(10.9%),灌注次数≥6次6例(2.2%)。
近75%的膀胱癌患者为NMIBC,TURBT及术后膀胱内灌注化疗/免疫治疗是治疗NMIBC的主要手段[1]。BCG用于预防NMIBC术后复发具有显著的疗效,在预防表浅性膀胱癌特别是T1G3肿瘤和原位癌TURBT术后复发方面,其疗效与丝裂霉素C相当,优于阿霉素、表阿霉素等常规膀胱灌注化疗药物[4]。1990年BCG膀胱内灌注治疗被批准用于治疗NMIBC,目前仍然是中、高危NMIBC术后辅助治疗的首选方案。
本研究结果显示,106例NMIBC患者TURBT术后辅助BCG膀胱灌注治疗,1年无复发生存率为91.5%,随访期间2例出现肿瘤进展。EORTC研究[7]纳入了1 812例BCG维持治疗1~3年的Ta~T1期膀胱癌患者,其1、5年复发率分别为25.9%(95%CI23.8~27.9)、41.3%(95%CI39.0~43.7);其中复发患者根据BCG治疗后复发时间分为早期复发(无病间期<4.5个月)和迟发复发(无病间期>4.5个月),结果显示肿瘤分级G1级、肿瘤数目<4个、肿瘤复发频率<1次/年的早期复发率为8%,而肿瘤分级G2/G3级、肿瘤数目>4个、复发频率>1次/年的早期复发率为29%;在迟发复发患者中,肿瘤数目<4个、复发频率< 1次/年患者和肿瘤数目>4个、复发频率>1次/年患者的1复发率分别为14.0%和33.0%,5年复发率分别为28.3%和51.7%。本研究结果与该报道中迟发复发患者结果相似,本研究多因素分析结果显示肿瘤复发病史是患者BCG治疗后再次复发的独立危险因素。本研究第一部分106例的1年无复发生存率为91.5%,略高于文献报道,9例BCG治疗后再次复发患者中,7例均为4.5个月以后复发。Brausi等[8]研究发现,无论肿瘤多发还是单发的膀胱癌患者,TURBT术后首次膀胱镜检查发现肿瘤复发,应考虑与首次TURBT质量相关,而并非肿瘤特异的生物学行为导致。国内关于NMIBC二次电切的专家共识也提出对部分患者首次TURBT术后(首次TURBT不充分;首次TURBT标本中无肌层组织等)进行二次电切,可以减少首次TURBT时肿瘤残留,并可对肿瘤进行精准分期和改善患者的预后[9]。本研究排除了切除范围不清、病理无法判断切除深度的病例,也许是本研究中复发患者以迟发复发为主,且1年复发率较低的主要原因。
EORTC-GU癌症协作组[5]统计了1 316例中、高危Ta、T1期膀胱癌患者的病例资料,经BCG膀胱灌注治疗后,出现局部不良反应826例(62.8%),全身不良反应403例(30.6%),局部或全身不良反应914例(69.5%)。其中发生率最高的局部不良反应是化学性膀胱炎460例(35.0%),最常见的全身不良反应是全身不适204例(15.5%)和发热106例(8.1%);103例(7.8%)因出现不良反应而终止治疗。
BCG膀胱灌注的不良反应发生率较低,严重不良反应发生率<5%,且大多数不良反应可得到有效的治疗[5,10]。BCG引起严重全身反应极为少见,BCG败血症发生率仅为0.4%[11],临床上多与灌注前插管致尿道损伤或膀胱尿道手术创面未完全愈合即行BCG灌注治疗相关。患者一旦诊断为BCG败血症,应予以足够重视。本研究结果显示,并发症发生率为64.1%,Ⅲ/Ⅳ级并发症发生率为4.0%,这些患者经过积极治疗均获得满意疗效。本研究中出现1例BCG灌注治疗后败血症患者,予三联抗结核药物、糖皮质激素及针对革兰阴性杆菌的抗菌药物后治愈。
EAU指南指出对于中、高危NMIBC患者来说,在降低肿瘤复发风险上,TURBT联合BCG膀胱灌注治疗效果优于单纯TURBT或TURBT联合膀胱内灌注化疗[12]。在中危及高危膀胱癌患者术后BCG治疗剂量选择上,CUETO研究[13]对比了标准剂量与1/3剂量BCG治疗NMIBC的疗效及药物相关不良反应,两方面均无明显差异。EAU指南中提及1/3治疗剂量的BCG可能是治疗中危膀胱癌的最小有效剂量。但是,EAU指南同时指出另有研究结果提示标准剂量BCG治疗对于多灶性肿瘤的效果更明显[14,15],而与标准剂量相比1/3治疗剂量的BCG治疗与肿瘤的高复发率有关,尤其在仅维持灌注1年的患者中[5]。在维持灌注时间的选择上,高危患者中标准剂量给药维持治疗3年的复发率低于1年;而中危患者中并没有这种差异,说明长时间的维持灌注能使复发风险高的患者受益[14]。因此,对于中危患者BCG治疗剂量及维持灌注时间要结合个体情况进行选择。目前尚无统一的BCG灌注方案,国内BCG常用剂量为60~120 mg,用40~50 ml生理盐水溶解,每周1次,共6周,后续可每2周维持灌注1次[16]。本研究中所有患者均选择标准治疗剂量,中位随访时间12个月,因此在剂量选择及结果方面未进行分层分析。
国内一项研究对36例NMIBC患者采用国产BCG膀胱灌注治疗,灌注后30例(83.3%)出现明显的尿频、尿急、尿痛等膀胱刺激症状,12例(33.3%)出现发热等流感样症状,11例(30.6%)出现血尿,经对症处理后症状均缓解[17]。国产BCG虽早在2013年就在国内上市,但在东北三省2014年以后才开始广泛使用,因此本研究随访时间较短,出现肿瘤进展的患者较少,且未出现死亡患者。本研究为回顾性研究,仍存在很多不可控因素,如手术操作、病理取材准确性、BCG灌注方式等,可能对数据分析有所影响。且对于疾病无复发生存期及无进展生存期仍需要长时间随访进一步评估。
综上所述,NMIBC患者TURBT术后应用BCG膀胱灌注治疗可获得较高的1年无复发生存率,灌注相关的Ⅲ/Ⅳ级并发症发生率较低,患者依从性好。
[1] Babjuk M, Burger M, Zigeuner R,et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013[J]. Eur Urol,2013,64:639-653.
[2] van Rhijn BW, Burger M, Lotan Y,et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy[J]. Eur Urol,2009,56:430-442.
[3] Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors[J]. J Urol,1976,116:180-183.
[4] Chou R, Selph S, Buckley DI,et al. Intravesical therapy for the treatment of nonmuscle invasive bladder cancer: a systematic review and meta-analysis[J]. J Urol,2017,197:1189-1199.
[5] Brausi M, Oddens J, Sylvester R,et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta,T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one3third dose with full dose and 1 year with 3 years of maintenance BCG[J]. Eur Urol,2014,65:69-76.
[6] Rischmann P, Desgrandchamps F, Malavaud B, et al. BCG intravesical instillations: recommendations for side-effects management[J]. Eur Urol. 2000,37
[7] Cambier S, Sylvester RJ, Collette L,et al. EORTC nomograms and risk groups for predicting recurrence,progression,and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance Bacillus Calmette-Guérin[J]. Eur Urol,2016,69:60-69.
[8] Brausi M, Collette L, Kurth K,et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies [J]. Eur Urol,2002,41:523-531.
[9] 中华医学会泌尿外科学分会,中国膀胱癌联盟. 非肌层浸润性膀胱癌二次电切中国专家共识[J].中华泌尿外科杂志,2017,38:561-563.
[10] van der Meijden AP, Sylvester RJ, Oosterlinck W,et al. Maintenance bacillus Calmette-Guerin for Ta,T1 bladder tumours is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase Ⅲ Trial[J]. Eur Urol,2003,44: 4293434.
[11] Lamm DL, van der Meijden PM, Morales A,et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer[J]. J Urol,1992,147:596-600.
[12] Babjuk M, B?hle A, Burger M,et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016[J]. Eur Urol,2017,71:447-461.
[13] Fernandez-Gomez J, Solsona E, Unda M,et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin:multivariate analysis of data from four randomized CUETO trials[J]. Eur Urol,2008,53:992-1001.
[14] Oddens J, Brausi M, Sylvester R,et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate-and high-risk Ta,T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance[J]. Eur Urol,2013,63:462-472.
[15] Martínez-Pi?eiro JA, Martínez-Pi?eiro L, Solsona E,et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial [J]. J Urol,2005,174(4
[16] 中华医学会泌尿外科学分会膀胱癌联盟.膀胱内灌注治疗操作规范(2015年版) [J].中华泌尿外科杂志,2015,36:481-483.
[17] 姜帅,郭剑明.基于单中心的国产卡介苗治疗非肌层浸润性膀胱癌临床应用分析[J].中国临床医学,2018,25: 409-411.

收藏此内容
推荐给朋友